Profile

Nationality : Indian
Keywords : Chirality, Optical resolution, Enantiomers, Diastereomeric salt formation, Solvent-induced chirality switching
Research advisor : Dr. Koichi Kodama
Why I entered GSC program
As an engineer with bio and chemical background, I was interested to pursue my research on optical resolution of enantiomers for emerging industrial applications. Furthermore, this program has been designed to meet our essential academic requirements which would help us to attain a challenging position in the field of science and technology with our technical and analytical skills.
Research title
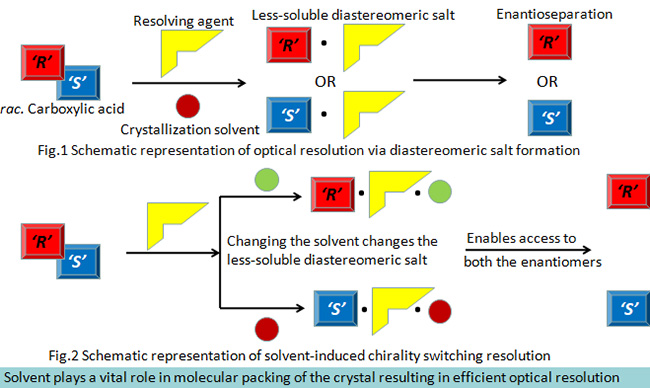
Optical resolution of carboxylic acids via diastereomeric salt formation to achieve solvent-induced chirality switching
Research abstract
A molecule is said to be chiral if it can exist as isomers (called enantiomers) that are non superimposable mirror images of each other. The significant application of chiral molecules in optically pure form as biologically active compounds, as additives for tailoring polymer properties and in opto-electronic devices reveals the importance of chirality and demands efficient methods for obtaining the compounds in an enantiomerically, highly pure form. Despite the available alternate techniques, optical resolution via diastereomeric salt formation remains the widely preferred method for obtaining pure enantiomers on an industrial scale owing to its simplicity, cost effectiveness and large-scale application. It is estimated that more than half of the chiral drugs in pharmaceutical industries were obtained by this method using enantiomerically pure resolving agents.On the other hand, ‘Solvent-induced chirality switching resolution’ is an effect in which the stereochemistry of the less-soluble salt changes depending on the solvent used for crystallization in optical resolution. In this research work, we propose to achieve solvent-induced chirality switching effect in the optical resolution of carboxylic acids via diastereomeric salt formation in order to access both the enantiomers of a target molecule in a highly efficient manner without changing the resolving agent.
Graphical abstract