Nishiyama Laboratory
Laboratory of Molecular Environmental Biology,
Department of Molecular Biology and Biochemistry
RESEARCH


Nishiyama Laboratory
Laboratory of Molecular Environmental Biology,
Department of Molecular Biology and Biochemistry
RESEARCH
Photosynthetic organisms have developed various mechanisms to cope with environmental stress. For example, when sunlight is strong or weak and when temperature rises or falls, photosynthetic organisms adjust their photosynthesis to the environmental changes and avoid injury caused by the environmental stress. They perceive changes in the environment, transmit the signals to the genome, and regulate the expression of various genes at the levels of transcription, translation, and post-translation.
In our laboratory, we are working on the mechanisms of the response of photosynthesis to environmental stress through the functions of genes and proteins in photosynthetic organisms, such as cyanobacteria, microalgae, and plants. We are also working on the production of biofuel and the conservation of marine resources toward the Sustainable Development Goals (SDGs).
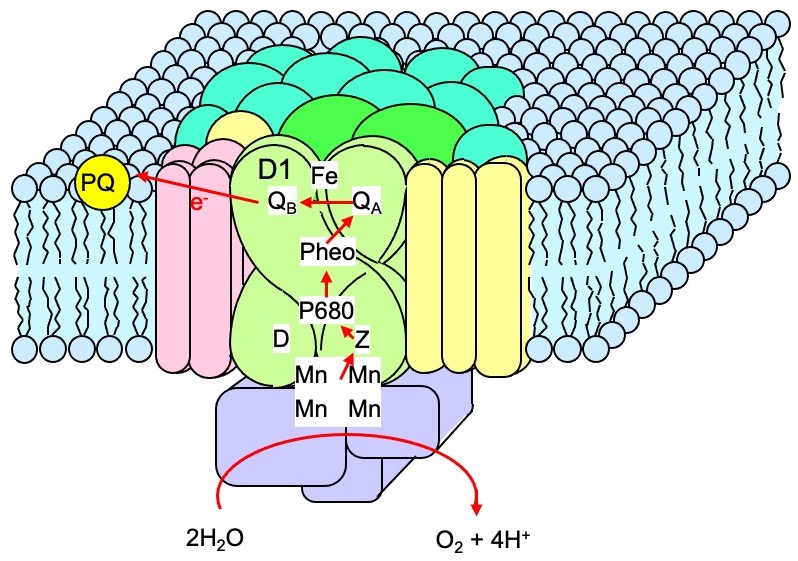
Photosystem II (PSII), a pigment-protein complex that converts light energy to chemical energy (Fig. 1), is sensitive to strong light. Inactivation of PSII by strong light is referred to as photoinhibition of PSII and is often associated with the suppression of the growth of photosynthetic organisms under strong light. Under strong light, reactive oxygen species (ROS) are abundantly produced in the photosynthetic machinery, causing oxidative stress.
We found that ROS accelerate the photoinhibition of PSII by inhibiting the repair of PSII. Furthermore, we found that ROS suppress the synthesis de novo of proteins that are required for the repair of PSII, such as the D1 protein, at the translational level. We are currently working on the molecular mechanism of the photoinhibition and repair of PSII with cyanobacteria, microalgae, and plants.
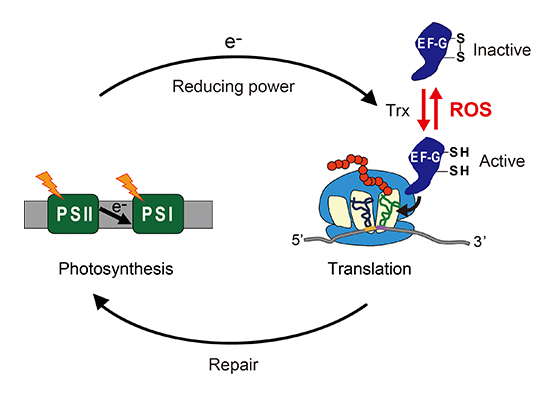
From our study of photoinhibition in cyanobacteria, we found that translation factors EF-G and EF-Tu are inactivated via oxidation of specific cysteine residues under oxidative conditions, with the resultant suppression of protein synthesis.
Thioredoxin was able to reactivate the oxidized EF-G and EF-Tu, suggesting that protein synthesis might be regulated by photosynthetic electron transport in a redox-dependent manner (Fig. 2). We are currently working on the mechanism of the redox regulation of protein synthesis as well as its physiological significance in cyanobacteria and plants.
Photosynthetic organisms can enhance the tolerance of photosynthesis to strong light by acclimation to strong light. We found that the ability to repair PSII is enhanced, together with accumulation of specific carotenoids, during acclimation to strong light (Fig. 3).
We also found that the enhanced repair ability was associated with light-induced expression of EF-Tu, with the resultant activation of protein synthesis. We are currently working on the mechanism of strong-light acclimation of photosynthesis in cyanobacteria and plants.

PSII is sensitive to high temperature and the thermal inactivation of PSII is often associated with thermal injury to the whole body of the organism. To cope with thermal injury, photosynthetic organisms can enhance the thermal tolerance of PSII via acclimation to moderately high temperatures.
We found that specific proteins and the turnover of lipids are required for the enhanced thermal tolerance of PSII during acclimation to high temperatures. We are currently working on the functions of these proteins and lipids in high-temperature acclimation, with a goal of generating high-temperature-tolerant plants.
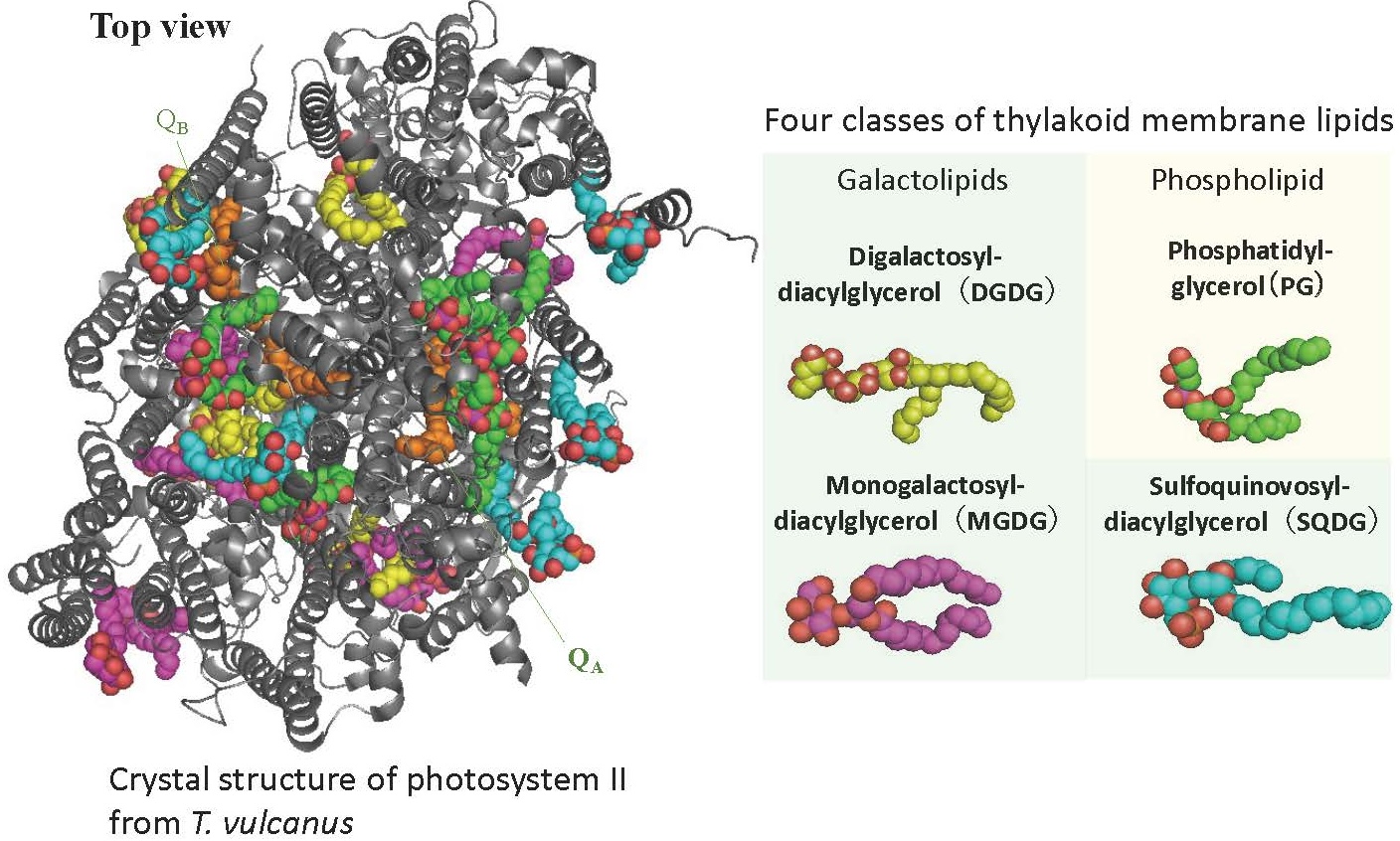
The thylakoid membranes are composed of four different glycerolipids (MGDG, DGDG, SQDG, and PG; Fig. 4).
These membrane lipids undergo a fast metabolic turnover, especially under environmental stress. Recently, we have shown that the detachment of fatty acids bound to membrane lipids by lipases is important for the repair of damaged photosystem II under strong light. We are currently investigating how the metabolic turnover of membrane lipids is related to photosynthesis by using cyanobacterial and plant mutants deficient in lipase genes and by chemical biology methods in which chemically modified artificial lipid molecules are incorporated into cells.
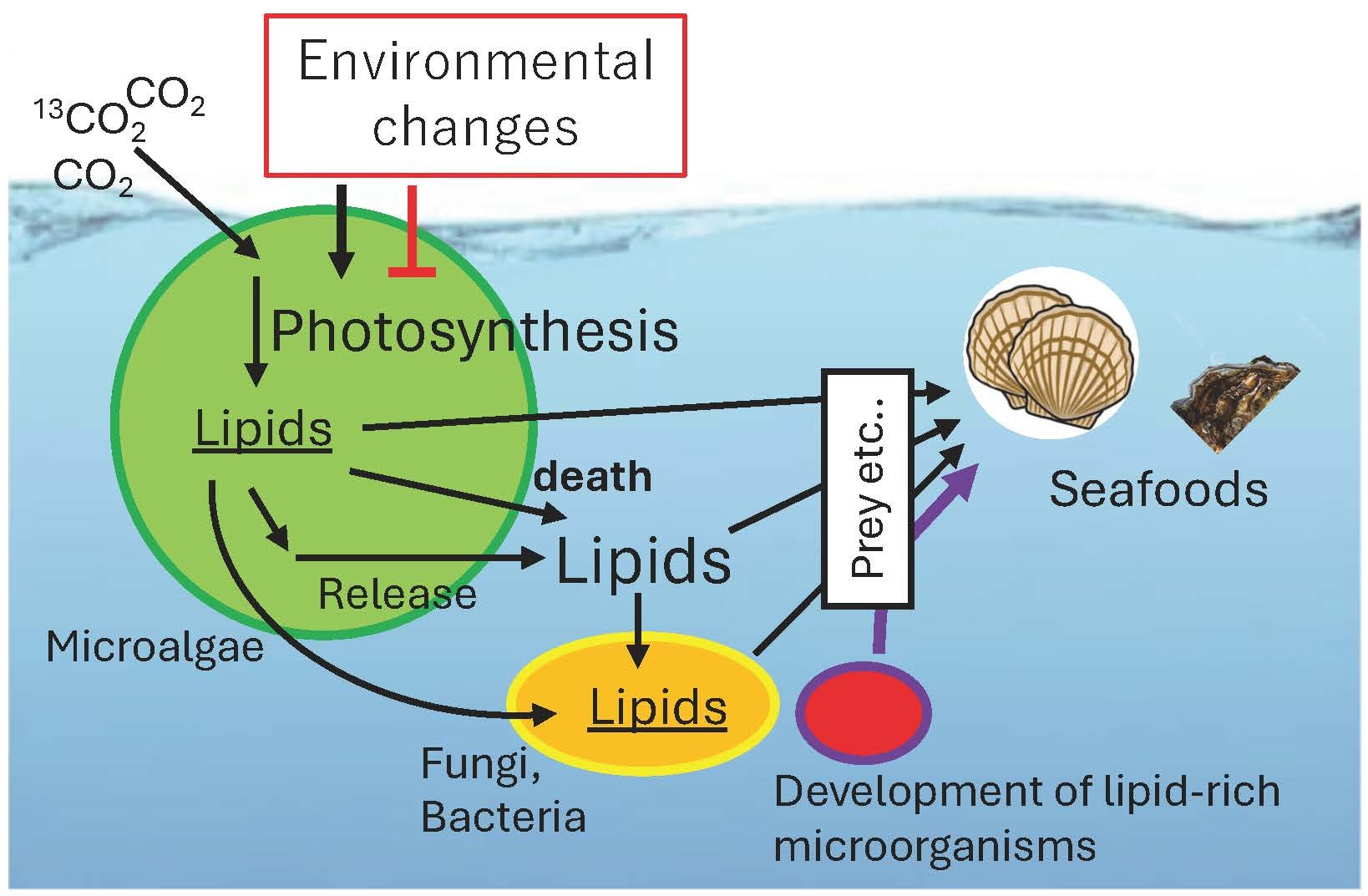
Microalgae such as green algae, diatoms and cyanobacteria are responsible for most of the photosynthetic activity on the Earth.
Fatty acids contained in these microalgae are transported through the ecosystem by predation by zooplankton or by cell death and rupture (Fig. 5).
In particular, polyunsaturated fatty acids synthesized by microalgae are often essential for survival for animals, and it remains unclear which pathways are used to transfer them from microalgae to which animals. We are currently working to clarify the metabolic dynamics of lipids in microbial ecosystems. This knowledge will improve the quality of cultured fish and shellfish and enable the isolation of novel lipid-requiring microorganisms in our future.
The rapid development of large blooms of microalgae is referred to as red tide, and the red tides of noxious species, such as the raphidophyte Chattonella antiqua and the dinoflagellate Karenia mikimotoi (Fig. 6), cause serious damage to aquaculture by killing fish, in particular, in the inner sea areas of Kyushu and Shikoku in Japan. However, the physiological activities, such as photosynthesis, of these noxious algae remain to be clarified.
We found that in K. mikimotoi, the photoinhibition of PSII is correlated with the decline of the red tides. In C. antiqua, the extracellular production of superoxide, which is a possible fish-killing factor, is regulated by photosynthesis and nutrient deficiency.
We are currently working on the mechanisms of the formation and decline of blooms and of the extracellular production of superoxide in these noxious algae. This study aims at establishing new technologies for red-tide prediction and protection of aquaculture from the red-tide-induced damage, contributing to the SDGs in terms of the conservation of marine resources and the stable supply of food via sustainable fishing.

Biofuel is a sustainable energy that will be substituted for fossil fuels. The production of biofuel with microalgae has been intensively studied, but a major factor that limits the fuel production is a decrease in the efficiency of photosynthesis, due to photoinhibition, during cultivation of microalgae under strong sunshine in natural environments.
We have developed various methods, including genetic manipulation, to improve the tolerance of photosynthesis to strong light and are currently applying these methods to biofuel-producing microalgae for more efficient and stable production of biofuel (Figs. 7 and 8). This study also aims at contributing to the sustainable energy production of the SDGs.