Nishiyama Laboratory
Laboratory of Molecular Environmental Biology,
Department of Molecular Biology and Biochemistry
RESEARCH


Nishiyama Laboratory
Laboratory of Molecular Environmental Biology,
Department of Molecular Biology and Biochemistry
RESEARCH
Photosynthetic organisms have developed various mechanisms to cope with environmental stress and thrive in a constantly changing environment. For example, they adjust their photosynthetic functions in response to varying light and temperature conditions to optimize their performance and avoid damage. To achieve this, they sense environmental changes, transmit signals, and precisely regulate the expression of specific genes at the transcriptional, translational, and post-translational levels.
In our laboratory, we study the mechanisms of photosynthetic environmental responses at the gene and protein levels in a range of photosynthetic organisms, including cyanobacteria, eukaryotic algae, and plants. We also develop microalgae-based biofuels and investigate the bloom dynamics and fish toxicity of harmful red-tide algae.
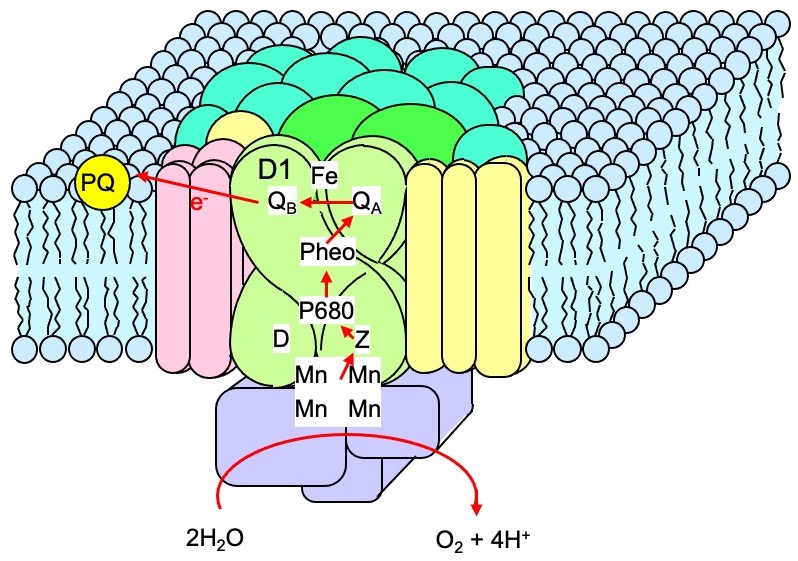
Photosystem II (PSII), a pigment-protein complex that converts light energy into chemical energy (Fig. 1), is sensitive to strong light. The light-induced inactivation of PSII is known as photoinhibition of PSII, which often suppresses the growth of photosynthetic organisms. Under strong light, the photosynthetic machinery produces abundant reactive oxygen species (ROS), leading to oxidative stress.
We have discovered that ROS accelerate the photoinhibition of PSII by inhibiting the repair of PSII. Specifically, we found that ROS suppress the de novo synthesis of proteins essential for PSII repair, such as the D1 protein, at the translational level. Our current research focuses on the molecular mechanisms of PSII repair during photoinhibition in cyanobacteria, microalgae, and plants.
Our research on photoinhibition in cyanobacteria revealed that under oxidative stress, the translational elongation factors EF-G and EF-Tu are inactivated through the oxidation of specific cysteine residues. This inactivation, in turn, suppresses protein synthesis.
We found that thioredoxin can reactivate the oxidized EF-G and EF-Tu, suggesting that protein synthesis is regulated by photosynthetic electron transport in a redox-dependent manner (Fig. 2). We are currently investigating the molecular mechanism and physiological significance of this redox regulation of protein synthesis in both cyanobacteria and plants.

Photosynthetic organisms can enhance the tolerance of photosynthesis to strong light through acclimation to strong light. We found that the ability to repair PSII is enhanced, along with the accumulation of specific carotenoids, during this process (Fig. 3).
We also found that the enhanced repair ability is related to light-induced EF-Tu expression, which subsequently activates protein synthesis. We are currently investigating the mechanism of strong-light acclimation of photosynthesis in cyanobacteria and plants.

PSII is sensitive to high temperatures, and its thermal inactivation is often associated with heat injury to the entire organism. To cope with this, photosynthetic organisms can enhance the thermal tolerance of PSII by acclimating to moderately high temperatures.
We have found that specific proteins and lipid turnover are essential for the enhanced thermal tolerance of PSII during high-temperature acclimation. Our current research focuses on the functions of these proteins and lipids, with the aim of creating heat-tolerant plants.
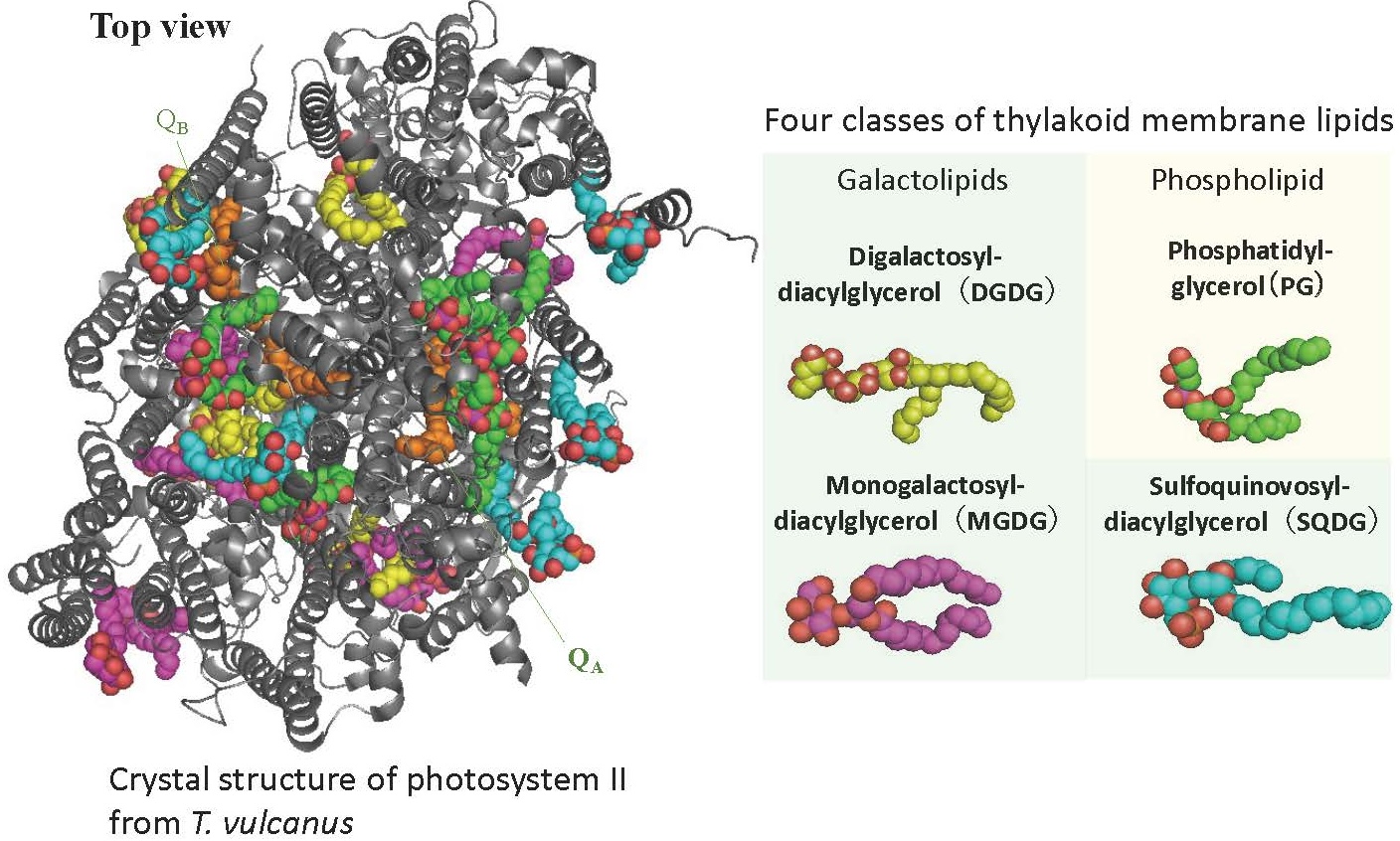
The thylakoid membranes are composed of four different glycerolipids: MGDG, DGDG, SQDG, and PG (Fig. 4).
These membrane lipids undergo a fast metabolic turnover, especially under environmental stress. We have recently shown that the detachment of fatty acids from membrane lipids by lipases is crucial for repairing damaged PSII under strong light. We are now investigating the relationship between membrane lipid turnover and photosynthesis. To do this, we were using cyanobacterial and plant mutants with defective lipase genes, as well as chemical biology approaches where we incorporate chemically modified artificial lipid molecules into cells.
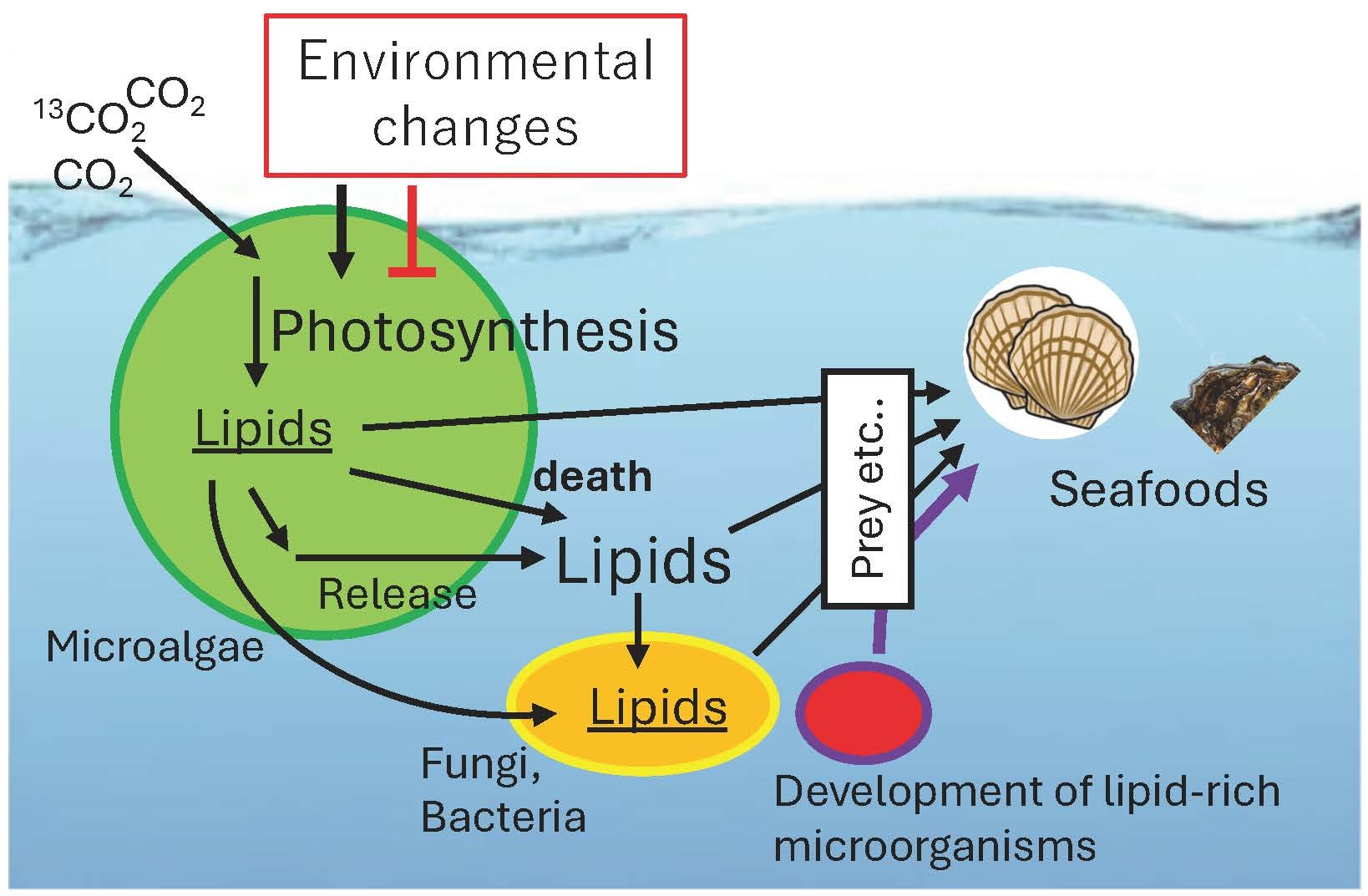
Microalgae, such as green algae, diatoms, and cyanobacteria, are responsible for most of the Earth's photosynthetic activity. Fatty acids from these microalgae are transported through the ecosystem via predation by zooplankton or through cell death and rupture (Fig. 5).
Fatty acids contained in these microalgae are transported through the ecosystem by predation by zooplankton or by cell death and rupture (Fig. 5).
In particular, polyunsaturated fatty acids synthesized by microalgae are often essential for animal survival. However, the exact pathways by which these fatty acids are transferred from microalgae to specific animals remain unclear. Our current research aims to clarify the metabolic dynamics of lipids within microbial ecosystems. This knowledge will not only help improve the quality of farmed fish and shellfish but also facilitate the isolation of novel lipid-requiring microorganisms in the future.
The rapid development of large microalgal blooms is commonly referred to as a red tide. Red tides caused by harmful species, such as the raphidophyte Chattonella antiqua and the dinoflagellate Karenia mikimotoi (Fig. 6), inflict serious damage on aquaculture by killing fish, particularly in the enclosed sea areas of Kyushu and Shikoku in Japan. However, key physiological activities, such as photosynthesis, of these harmful algae have yet to be fully clarified.
We found that in Karenia mikimotoi, the photoinhibition of PSII is correlated with the decline of red tides. We also found that in Chattonella antiqua, the extracellular production of superoxide, a potential fish-killing factor, is regulated by both photosynthesis and nutrient deficiency.
We are currently investigating the mechanisms underlying the formation and decline of these harmful algal blooms, as well as their extracellular production of superoxide. Our study aims to establish new technologies for predicting red tides and protecting aquaculture from the damage they cause. This research will contribute to the conservation of marine resources and ensure a stable food supply through sustainable fishing practices.

Biofuels are a sustainable energy source that will replace fossil fuels. The production of biofuels using microalgae has been intensively studied, but a major limiting factor is the decrease in photosynthetic efficiency caused by photoinhibition. This typically occurs when microalgae are cultivated under strong sunlight in natural environments.
We have developed various methods, including genetic engineering, to improve the tolerance of photosynthesis to strong light. We are now applying these methods to biofuel-producing microalgae to achieve more efficient and stable biofuel production (Figs. 7 and 8). This study is also aimed at contributing to sustainable energy production.